Glycosylation: Impact on Health and Disease


What are Glycans?
Glycans are essential for all life. These are long-chain sugars that are present on proteins, lipids (also known as fats) and nucleic acids, and which make these biomolecules functional. When we think of sugars, our first thought may be about the sugars from food we eat, such as glucose and sucrose.
Glycans can be built from up to 9 types of sugar building blocks which can be connected to form a variety of branched structures. The versatility of these building blocks means that most of them can be found in all living organisms, from plants to Homo sapiens. Glycans have a primary role in making cells and proteins functional. Without glycans, many bodily processes would not occur.
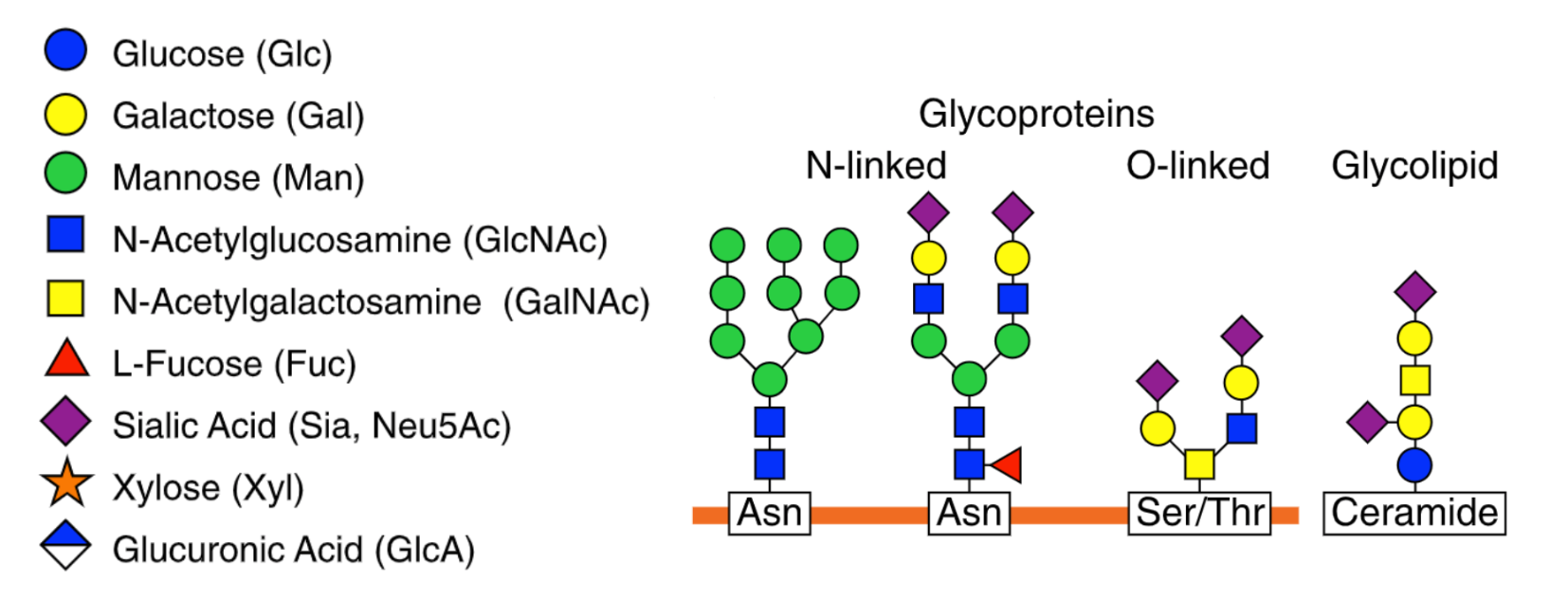
What is Glycosylation?
Glycosylation is the process by which glycan molecules are chemically attached to proteins or lipids to form final products known as glycoproteins or glycolipids, respectively. One common example of a glycosylated protein is immunoglobulin G (IgG).
IgG antibodies neutralise pathogens and viruses, exerting these functions via glycans that coat them. Without glycans, IgG antibodies would not be able to protect us from dangerous bugs. Think of glycosylation as adding icing to a cake. Just as icing enhances the appearance and taste of a cake, glycosylation modifies proteins, altering their structure and function in various ways. This highly regulated process takes place towards the end of a protein being created within the cell.
Importance of Glycosylation
How our proteins are created and look is encoded in our DNA, but how they function depends on glycans. Glycosylation is also vital for adequate cell signalling processes, preventing the unwanted clustering of proteins and facilitating immune response.
Want to know more about the functional wonders of glycans? On a systemic level, glycans are essential for defining blood groups. The presence or absence of different glycan structures dictates blood type and compatibility for transfusions. Glycans also play a critical role in fertilisation by facilitating the recognition and binding between the sperm and egg.
Without this essential process, proteins would not have their proper shape and would not be able to interact with other molecules, potentially leading to disruptions in cellular processes and health issues. In fact, changes in glycosylation are seen in diseases, including cancer, as well as autoimmune, infectious and other chronic inflammatory diseases.
What regulates Glycosylation?
Despite glycosylation being a complex process that is not yet fully understood, it is known to be carefully regulated by a series of enzymes which add or modify the glycans on the protein in question. There are various subtypes of glycosylation depending on the type of glycan and which portion of the protein the glycans are added.
Main Types of Glycosylation
N-Linked Glycosylation
Amino acids are the building blocks of proteins, with the human body using 20 different amino acids to create all proteins. N-linked glycosylation is a process that involves the attachment of an entire (big or small) of an N-linked glycan to the specific amino acid asparagine residue within a protein. The ‘N’ in N-linked refers to the glycans being attached to the nitrogen atom of the asparagine.
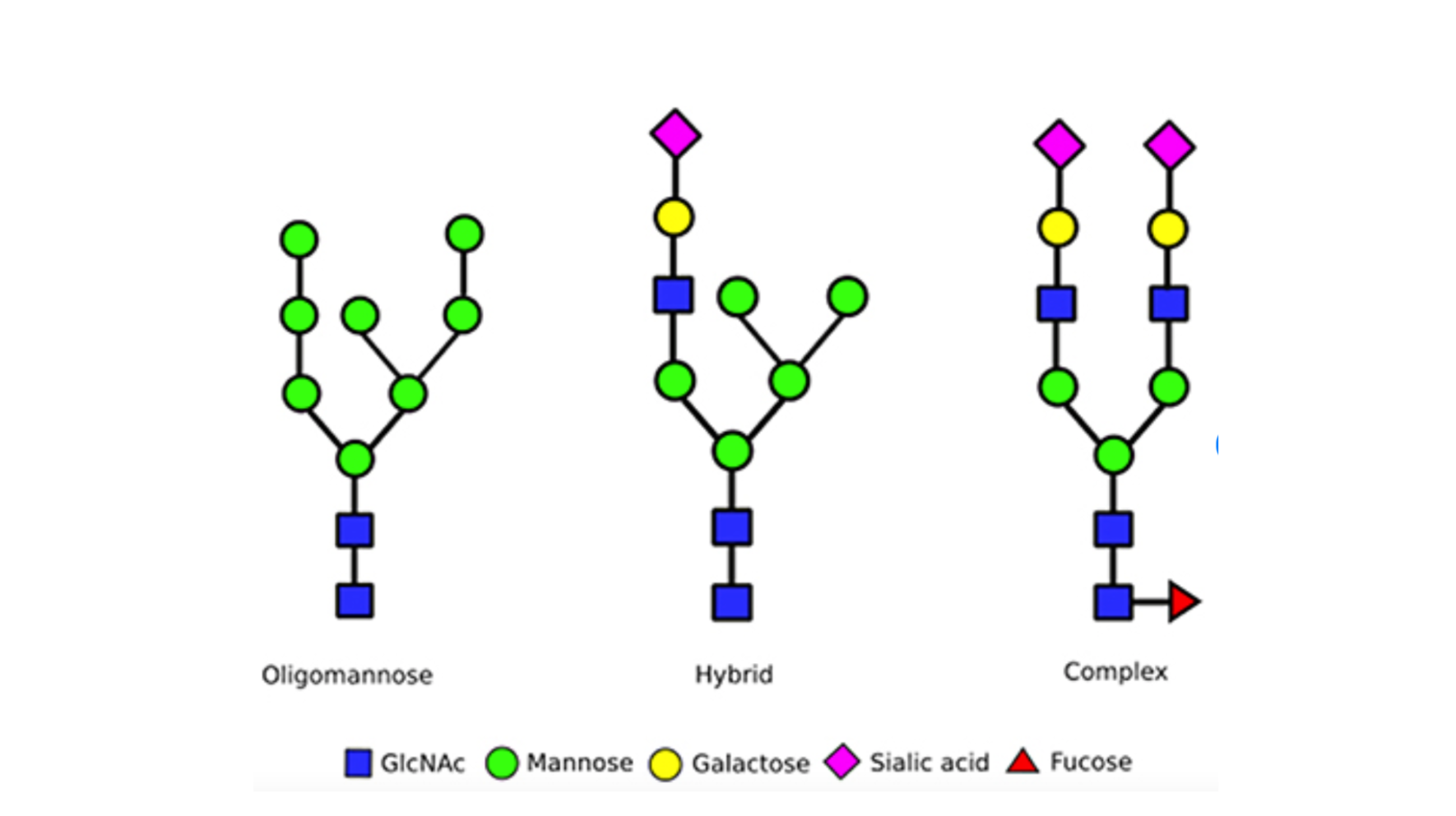
There are 3 primary types of N-linked glycans that are commonly found in humans, these differ based on the number of branches and the types of sugar blocks they are built from:
- Oligomannose glycans are made of mannose sugar units attached to a core of two N-acetylglucosamine (GlcNAc) units and three mannoses. Oligomannose glycans are relatively simple and uniform in structure.
- Complex glycans contain a core of 2 GlcNAc units and three mannoses but can have other sugar building blocks such as sialic acid, galactose, and additional GlcNAc units. Complex glycans can have multiple branching points and varied structures, making them highly diverse.
- Hybrid glycans have features of both oligomannose and complex glycans. They typically have at least one branch that resembles oligomannose and another that is more complex, often including additional types of sugars.
N-linked glycosylation usually takes place in special compartments of cells called the endoplasmic reticulum (ER) membrane and Golgi apparatus and comprises of 3 main stages:
- The creation of the lipid-linked oligosaccharide (LLO) precursor: Think of this precursor as the scaffold and transporter to build the N-glycan complex. A series of enzymes add different sugar molecules to this precursor.
- The Assembly of N-linked glycan: In this complex stage, a series of enzymes known as glycosyltransferases, continues to add sugar molecules to the LLO precursor, eventually leading to the formation of the N-linked glycan chain.
- The transfer of the N-linked glycan to the protein: In this step, an enzyme called oligosaccharyltransferase (OST), transfers the N-linked glycan from the LLO precursor to the asparagine residue within a protein molecule. The protein then continues to get modified as it travels through the ER and Golgi.
N-linked glycosylation is best known for ensuring the protein folds correctly into its final form, ensuring it is able to function.
O-Linked Glycosylation
O-linked glycosylation focuses on the addition of glycans to the oxygen atom of amino acid residues serine or threonine within a protein. This process takes place in the Golgi apparatus after the protein has been formed and consists of 3 stages:
- Initiation: A single sugar molecule is attached to the serine or threonine residues by enzymes called O-GalNAc transferases.
- Elongation: Following the initial attachment of a single sugar, the glycan chain can undergo further elongation through the addition of additional sugar residues. This process involves the activity of various enzymes that add different sugar molecules to the growing glycan chain. The enzymes that support this elongation depend on the cell or tissue type.
- Branching: in certain cases, O-linked glycans can become a branched structure to change the function of the glycoprotein. This is done through the addition of sugar molecules by branching enzymes.
O-glycosylation can influence various protein aspects, such as enhancing protein stability. Additionally, O-linked glycosylation also impacts cell signalling which impacts cellular migration and immune cell recognition.
GPI Anchor Glycosylation
Whilst N-linked and O-linked glycosylation are the two most distinguishable types, another form of glycosylation includes Glycosylphosphatidylinositol (GPI) anchor glycosylation. Unlike N- or O-linked glycosylation, which directly attaches glycans to specific amino acids within a protein, a glycolipid anchor is used to attach to specific proteins on the cell surface. This is important as it allows for cellular signalling and cell-to-cell communication to occur.
Glycosylation vs Glycation
Glycosylation often gets confused with glycation, which although sounds similar, is a distinct biological process.
Glycation, the random and non-enzymatic attachment of sugars to proteins and lipids, contrasts with enzymatically controlled glycosylation, where glycans are deliberately added to proteins. Up to 70% of human proteins go through the process of being glycosylated.
Glycation results in the production of advanced glycation end products (AGEs), which can later bind to receptors known as receptor of advanced glycation end products (RAGEs), found on various cell types including immune cells. This can result in inflammation and oxidative stress and potentially contribute to chronic disease development.
For instance, HbA1c is a common diabetes biomarker and is an example of a glycated protein haemoglobin that is responsible for carrying oxygen in red blood cells; it also denotes glucose molecules binding to haemoglobin. Glycation of haemoglobin unregulated process occurs when blood glucose levels are elevated, with HbA1c levels serving as indicators of blood glucose control for up to 3 months before the measurement
The table below highlights the main differences between glycosylation vs glycation.
Glycosylation in Health and Disease
Since glycosylation has a crucial role in impacting the structure, stability and function of proteins all over the body, this can have major implications on various aspects of our health. We will delve deeper to explore how glycosylation can impact:
- Immunity and Inflammation
- Congenital Disorders of Glycosylation (CDG)
- Glycosylation and Cancer
Immunity and Inflammation
In immunity, inflammation is a fundamental biological response that helps our body against all forms of infection, injury and disease. However, inflammation must be a tightly regulated process that begins and ends in a timely manner to gain maximum benefits.
Acute inflammation is self-limiting and is initiated at the location of injury or infection, resulting in the release of chemical messengers known as pro-inflammatory cytokines. This helps facilitate wound healing and provides an effective defence against pathogens.
However, chronic inflammation can last for weeks, months or indefinitely. In this undesirable scenario, inflammation becomes the problem rather than the solution, as chronic inflammation can lead to immune system dysfunction by causing a persistent release of pro-inflammatory cytokines. This can result in the breakdown and damage of healthy tissue in a misdirected attempt at repair and healing.
Many diseases are characterised by chronic inflammation, including but not limited to:
- Diabetes
- Coronary Heart Disease (e.g. atherosclerosis)
- Rheumatoid Arthritis
- Systemic lupus erythematosus
In the 1980s, the first IgG glycosylation abnormalities were discovered in patients with rheumatoid arthritis, with research highlighting patients presented with fewer galactose and sialic acid (which has an anti-inflammatory effect) and greater fructose and branching. This highlights specific changes in glycosylation are a defining feature of chronic inflammation.
Congenital Disorders of Glycosylation
Congenital disorders of glycosylation (CDGs) are a large group of rare genetic disorders that affect glycosylation. As the healthy function of cells heavily relies on correct glycosylation, individuals with CDGs usually have a wide range of health issues.
Many different organs or entire systems may be affected. Presentation may include neurological phenomena such as developmental delay and reduced muscle tone, failure to thrive, or blood disorders and liver dysfunction.
In the majority of CDG cases, there may be autosomal recessive patterns of inheritance. This means that a child inherits two copies of a mutated gene, one from each parent and this will be expressed. However, there are also autosomal dominant patterns of inheritance, where a child needs just one copy of a mutated gene from a single parent which is enough to be expressed.
Certain forms of CDG may be broadly identified with a blood test to detect abnormal glycans. Once a glycosylation defect is found, additional tests such as molecular testing must be done to confirm and identify the specific CDG subtype.
There is no known cure for CDGs, but treatments are available to manage symptoms and improve the quality of life. Due to the different forms of CDG, their varying symptoms and severity, the treatment plan for each individual can be unique. It can consist of:
- Therapy to support feeding, speech or physical needs
- Plasma infusions or blood thinning medication to address blood clotting issues
- Medications for thyroid or epilepsy issues.
Glycosylation and Cancer
Cancer is the uncontrollable growth of cells and can spread to other parts of the body. It is also one of the leading causes of death worldwide, accounting for nearly 10 million deaths in 2020 alone, or nearly one in six deaths. The most common types of cancers include breast, lung, colon, rectum and prostate cancers. Now more than ever, the need for key biomarkers that can help determine the risk of developing cancer are needed.
Glycosylation can be altered in cancer, which can not only directly impact cell growth and survival, but also help cancer cells evade detection by the immune system. The major alterations in cancers include alterations to N-linked glycans, O-linked glycans and the expression levels of key glycosylation enzymes such as glycosyltransferases.
This not only impacts glycan structure and function but also the biological activity of a cell, such as cell signalling and immune detection. By understanding these changes in glycosylations, we can utilise glycans as biomarkers for cancer.
For instance, hepatocellular carcinoma is the most common type of liver cancer. It is commonly screened for using ultrasound with a blood test measuring elevated levels of a specific biomarker called alpha-fetoprotein (AFP).
However, this glycoprotein is associated with false-positive results, as AFP levels may rise due to conditions like chronic hepatitis and liver cirrhosis. The introduction of AFP-L3, a specific glycoprotein subfraction of AFP, marks a significant advancement.
Approved by the Food and Drug Admission (FDA) for use in cancer diagnosis, AFP-L3 enhances the early detection of hepatocellular carcinoma (HCC). It more accurately distinguishes between cancerous growths and non-cancerous liver disease, thereby improving the efficacy of detection.
Conclusion
In summary, glycosylation is a crucial process without which there would be no life. Glycosylation of proteins specifically allows them to function properly. This is especially important in the context of our immune system which relies on glycans to shield us from external pathogens and other potential triggers of chronic inflammation.
Understanding glycosylation is necessary to understand the causes of disease as well as improve disease prediction and treatment. So whilst glycosylation might be a confusing topic, it holds the key to unlocking important insights into our health and well-being.
GlycanAge is a powerful biological age test that can assess the status of your body's chronic inflammation by paying attention to the N-linked glycans attached to your IgG antibodies. By doing this, GlycanAge can assess an individual’s biological age, which can differ from their chronological age.
With just a simple at-home blood testing kit, GlycanAge can assess an individual’s physiological age, which may differ from their chronological age. Understanding the inflammatory status of your body’s immune system through these results allows for sustainable lifestyle alterations in areas such as diet, sleep, stress and exercise. This proactive approach can not only help you live a longer life but a healthier life.

Start or continue your GlycanAge journey
Don’t be afraid to reach out to us and ask questions, provide commentary or suggest topics.
 GlycanAge is a biological age test paired with expert advice to help guide your wellness.
GlycanAge is a biological age test paired with expert advice to help guide your wellness.